Abstract
Background:
Clinical outcomes for children with FLT3-mutant AML and infants with KMT2A-rearranged (KMT2A-R) B-ALL remain dismal. These leukemias share a common feature of aberrant activation of FLT3 kinase signaling, which occurs by activating FLT3 mutations in AML and by overexpression of wild-type FLT3 in KMT2A-R ALL. Several FLT3 tyrosine kinase inhibitors (FLT3i) are approved for adults with FLT3-mutant AML, but potential efficacy against KMT2A-R ALL remains incompletely characterized and may differ from responses in AML. We previously developed and preclinically validated chimeric antigen receptor (CAR) T cells directed against FLT3 (FLT3CART), which importantly showed potent anti-leukemia activity in preclinical models of both childhood FLT3-mutant AML and infant KMT2A-R ALL (Chien CD et al. ASH 2016). In the current studies, we hypothesized that combinatorial targeting of these two high-risk leukemia subtypes with FLT3CART and the selective next-generation FLT3i gilteritinib would have superior activity and potentially mitigate therapeutic resistance now known to occur with kinase inhibitors or CAR T cell immunotherapy.
Methods and Results:
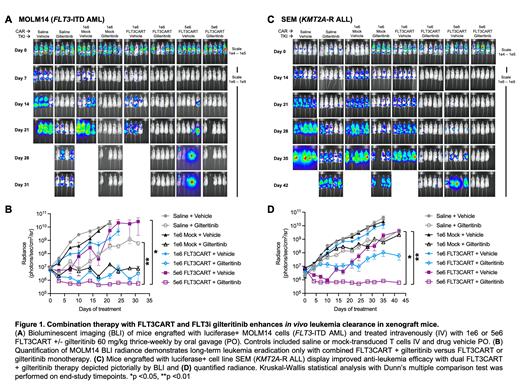
We first assessed in vitro sensitivity of human FLT3-mutant AML and KMT2A-R ALL cell lines to gilteritinib, a second-generation selective FLT3i with established clinical activity in FLT3-mutant AML and unknown activity in KMT2A-R ALL. As detrimental effects of kinase inhibitors (e.g., dasatinib, ruxolitinib) upon CAR T cells have been reported, we evaluated for similar effects with gilteritinib co-incubated in vitro with CD3/CD28-bead activated healthy human donor T cells. However, we observed minimal deleterious effects of gilteritinib on normal T cell viability, immunophenotype, and IL-2 and interferon-gamma (IFNg) production. We validated combinatorial effects of gilteritinib and FLT3CART-induced cytotoxicity against FLT3-mutant AML and KMT2A-R ALL cell lines in vitro without impairment of IL-2/IFNg production. We then assessed this dual therapy approach in luciferase+ FLT3-mutant AML (MOLM14) and KMT2A-R ALL (SEM) cell line murine xenograft models. As predicted, both FLT3CART and gilteritinib monotherapies transiently inhibited in vivo leukemia proliferation, although leukemia progression eventually occurred. Conversely, FLT3CART and gilteritinib combination therapy strikingly induced enhanced and sustained leukemia clearance in all assessed AML and ALL cell line xenograft models (Figure 1). Confirmatory studies in our established childhood FLT3-mutant AML and KMT2A-R ALL patient-derived xenograft (PDX) models have also demonstrated potent anti-leukemia efficacy of combined FLT3CART and gilteritinib therapy.
Earlier-generation FLT3i have been reported to increase cell surface FLT3 expression on FLT3-mutant AML cells. Given the known importance of target antigen site density for CAR T cell efficacy, we reasoned that a sequential approach to dual therapy with FLT3i 'priming' followed by FLT3CART may be superior to a simultaneous treatment strategy. In vitro studies with leukemia cell lines and in vivo studies with PDX models indeed confirmed gilteritinib-induced increases in FLT3 surface antigen density in FLT3-mutant AML cells. Intriguingly, we observed contrasting effects in KMT2A-R ALL cell lines and PDX with decreased surface FLT3 expression upon gilteritinib exposure. Ongoing studies are currently validating gilteritinib priming for FLT3CART given these initial data suggesting potentially divergent sequencing approaches in FLT3-mutant AML versus KMT2A-R ALL.
Conclusions:
Taken together, our preclinical studies demonstrate that dual targeting with FLT3CART immunotherapy and gilteritinib is a promising therapeutic strategy in FLT3-mutant AML and, importantly, also in KMT2A-R ALL. Notably, we also report minimal negative effects of gilteritinib on FLT3CART, suggesting that FLT3i may be used to enhance CAR T cell immunotherapy without inhibiting T cell function. Phase 1 clinical trials of FLT3CART will open soon for adults and children with FLT3-mutant AML and/or KMT2A-R ALL.
Fry: Sana Biotechnology: Current Employment, Current equity holder in publicly-traded company; ElevateBio: Research Funding. Tasian: Kura Oncology: Consultancy; Aleta Biotherapeutics: Consultancy; Gilead Sciences: Research Funding; Incyte Corporation: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal